When a pharmacist hands you a pill bottle with a different name than what your doctor wrote, you might wonder: is this really the same thing? It’s not just branding. It’s science, law, and decades of regulatory rigor behind every generic drug substitution. Pharmacists don’t guess. They don’t rely on memory. They follow a strict, science-backed process to confirm that a generic version is therapeutically identical to the brand-name drug you were prescribed.
The Legal Foundation: Hatch-Waxman and the Orange Book
The system pharmacists use today started in 1984 with the Hatch-Waxman Act. Before this, generic drugs had to go through the same expensive, time-consuming clinical trials as brand-name drugs. That made generics rare and expensive. The law changed that by creating the Abbreviated New Drug Application (ANDA) pathway. Now, generic manufacturers don’t need to prove the drug works again-they just need to prove it works the same way.
To make that possible, the U.S. Food and Drug Administration (FDA) created the Approved Drug Products with Therapeutic Equivalence Evaluations, better known as the Orange Book. First published in 1980, it’s updated monthly and is the single legal authority pharmacists use to decide if a generic can be swapped for a brand. It’s not a suggestion. It’s the rulebook.
Every drug in the Orange Book gets a two-letter code. If you see ‘AB’, that’s the gold standard. It means the generic has been proven to have the same active ingredient, strength, dosage form, and-most importantly-the same rate and amount of absorption in the body as the brand. About 98.7% of rated products carry this rating. If you see ‘B’, that means the FDA hasn’t found it equivalent. No substitution is allowed.
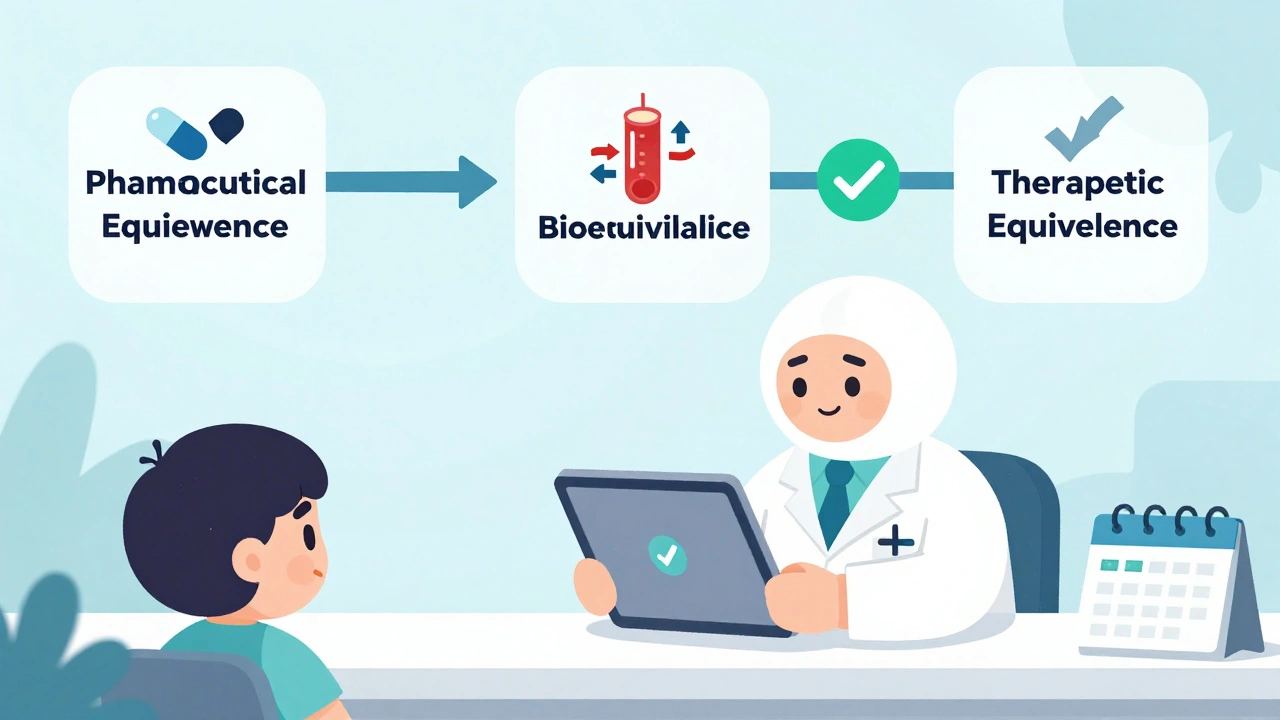
Three Layers of Equivalence: What Pharmacists Check
Verifying equivalence isn’t one check-it’s three stacked layers.
First: Pharmaceutical Equivalence. The generic must contain the exact same active ingredient, in the same strength, and in the same form-tablet, capsule, injection, etc. A 10mg amoxicillin capsule from a generic maker must match the brand in every physical way. This is checked against FDA-approved labeling.
Second: Bioequivalence. This is where science gets precise. The generic must deliver the same amount of drug into your bloodstream at the same rate as the brand. How? Through controlled studies in healthy volunteers. The FDA requires that the 90% confidence interval for the ratio of the generic’s maximum concentration (Cmax) and total exposure (AUC) to the brand’s falls between 80% and 125%. That’s not a guess. It’s a statistically proven window that ensures no meaningful difference in effect.
For drugs with a narrow therapeutic index-like warfarin, levothyroxine, or phenytoin-the rules are tighter. The acceptable range can shrink to 90-111%. That’s because even a small difference in blood levels can cause harm or reduce effectiveness.
Third: Therapeutic Equivalence. This is the final call. The FDA combines pharmaceutical and bioequivalence data to assign the ‘A’ or ‘B’ rating. Only ‘A’-rated drugs can be substituted under state law. The Orange Book is the only source that gives this official determination.
How Pharmacists Use the Orange Book in Practice
Every day, pharmacists open the Orange Book-either through the free FDA mobile app, their pharmacy software, or the online version. The process takes 8 to 12 seconds per prescription. Here’s how it works:
- Find the brand-name drug prescribed.
- Look up its Reference Listed Drug (RLD) in the Orange Book.
- Check which generic products are listed as therapeutically equivalent (‘A’ rating).
- Confirm the generic’s active ingredient, strength, and dosage form match the prescription.
- Ensure the prescriber hasn’t written “Dispense as Written” or “Do Not Substitute.”
Pharmacists cross-check this with other databases like Micromedex or Lexicomp-but only as backups. The Orange Book is the only one that carries legal weight. A 2021 study found 99.3% of pharmacists rely on it as their primary tool.
What happens if a drug isn’t listed? That’s rare-only about 5.7% of substitutions fall into this category. In those cases, pharmacists follow FDA guidance: they don’t substitute unless there’s clear evidence of equivalence from published studies or direct FDA communication. They err on the side of caution.
Why This System Works-And Why It’s Trusted
Some patients worry generics aren’t as good. But the data says otherwise. A 2020 FDA meta-analysis of over 1.2 million prescriptions found no meaningful difference in adverse events between brand and generic drugs: 0.78% for brands, 0.81% for generics. That difference? Statistically meaningless.
Research from the Journal of Generic Medicines analyzed 2,147 bioequivalence studies and found that 97.8% of generics showed less than a 5% difference in total drug exposure (AUC) compared to the brand. Most were within 2-3%.
And the system is legally protected. In 2019, a Texas pharmacist was sanctioned for substituting a drug not listed in the Orange Book. The court ruled: the Orange Book is the law. Deviating from it opens pharmacists to liability.
Even critics acknowledge its strength. Dr. Randall Stafford of Stanford noted that complex products-like inhalers or topical creams-can be harder to evaluate with traditional blood tests. But he didn’t call for scrapping the system. He called for better tools. And the FDA is responding: as of 2024, they’ve issued product-specific guidances for 1,850 complex drugs to improve evaluation accuracy.
Training, Compliance, and the Future
Pharmacists don’t learn this on the job. Every new hire gets 2-4 hours of formal training on Orange Book use. Competency checks show 89.3% accuracy after training. That’s not luck. That’s structured education.
Today, 90.7% of all prescriptions in the U.S. are for generics. That’s over 8.9 billion prescriptions a year. Without a reliable verification system, that scale would be impossible-and dangerous.
The future brings new challenges. Biosimilars-generic versions of biologic drugs like Humira or Enbrel-are growing fast. But they’re not in the Orange Book. They’re in the Purple Book, which only lists 47 of 350 approved biosimilars as of mid-2024. Pharmacists are still figuring out how to verify these. The FDA has allocated $28.5 million through GDUFA III to develop better methods for complex drugs, including inhalers, topical steroids, and nasal sprays.
But the core remains unchanged: pharmacists verify equivalence by following the Orange Book. Not because it’s convenient. Not because it’s popular. Because it’s the only system scientifically validated, legally binding, and proven safe across billions of doses.

What Patients Should Know
If your pharmacist switches your medication, don’t panic. They’re not cutting corners. They’re following a rigorous, science-based protocol designed to keep you safe and save you money. The generic you get is not a cheaper version-it’s an identical one, tested and approved by the same agency that approved the brand.
If you have concerns, ask your pharmacist to show you the Orange Book rating. They’ll be happy to explain. Most patients don’t realize they’re getting the same medicine. But now you do.
Are generic drugs really the same as brand-name drugs?
Yes-when they’re rated ‘A’ in the FDA’s Orange Book. Pharmacists verify that generics have the same active ingredient, strength, dosage form, and bioequivalence as the brand. Studies show no meaningful difference in how they work in the body or in patient outcomes.
Why do some generics look different from the brand?
Generic manufacturers can’t copy the brand’s appearance due to trademark laws. So color, shape, or packaging may differ-but the active ingredient and how it’s absorbed are identical. The Orange Book confirms this, not the pill’s color.
Can pharmacists substitute any generic for a brand?
Only if the generic has an ‘A’ rating in the FDA Orange Book and the prescriber hasn’t written “Do Not Substitute.” In 49 U.S. states, substitution is automatic unless blocked. Massachusetts is the only exception where prescriber consent is always required.
What if a drug isn’t listed in the Orange Book?
If a generic isn’t listed, pharmacists cannot legally substitute it unless the FDA has issued specific guidance allowing it. In these rare cases, the pharmacist will dispense the brand or consult the prescriber. Never assume an unlisted product is equivalent.
Do pharmacists get training on the Orange Book?
Yes. All new pharmacists receive 2-4 hours of formal training on using the Orange Book. Pharmacy schools include it in their curriculum, and competency tests show 89.3% accuracy after training. It’s not optional-it’s required.
Is the Orange Book the only resource pharmacists use?
No-but it’s the only one that matters legally. Pharmacists may use Micromedex or Lexicomp for quick reference, but they must confirm the Orange Book’s ‘A’ rating before substituting. Using other sources alone can lead to legal consequences.
Are there drugs where generics aren’t safe to substitute?
For drugs with a narrow therapeutic index-like warfarin, levothyroxine, or cyclosporine-some prescribers prefer to stick with the brand. But the FDA still considers ‘A’-rated generics safe for these. The decision to substitute is up to the prescriber, not the pharmacist, unless the prescription allows substitution.
How often is the Orange Book updated?
The Orange Book is updated monthly with supplements and fully revised annually. Pharmacists are trained to check for the latest updates, especially since new generics are approved every week. Outdated information can lead to errors.
Next Steps for Patients and Pharmacists
For patients: If you’re unsure about a generic switch, ask your pharmacist to explain the Orange Book rating. They’re trained to do this-and they want you to feel confident.
For pharmacists: Stay current with monthly Orange Book updates. Use the FDA’s free mobile app. Document your verification steps. If a product isn’t listed, don’t guess-consult the prescriber or wait for FDA guidance.
The system works because it’s clear, consistent, and backed by data. It’s not perfect-but it’s the best we have. And for 90% of prescriptions, it’s keeping people healthy, safe, and saving billions every year.






Comments(15)