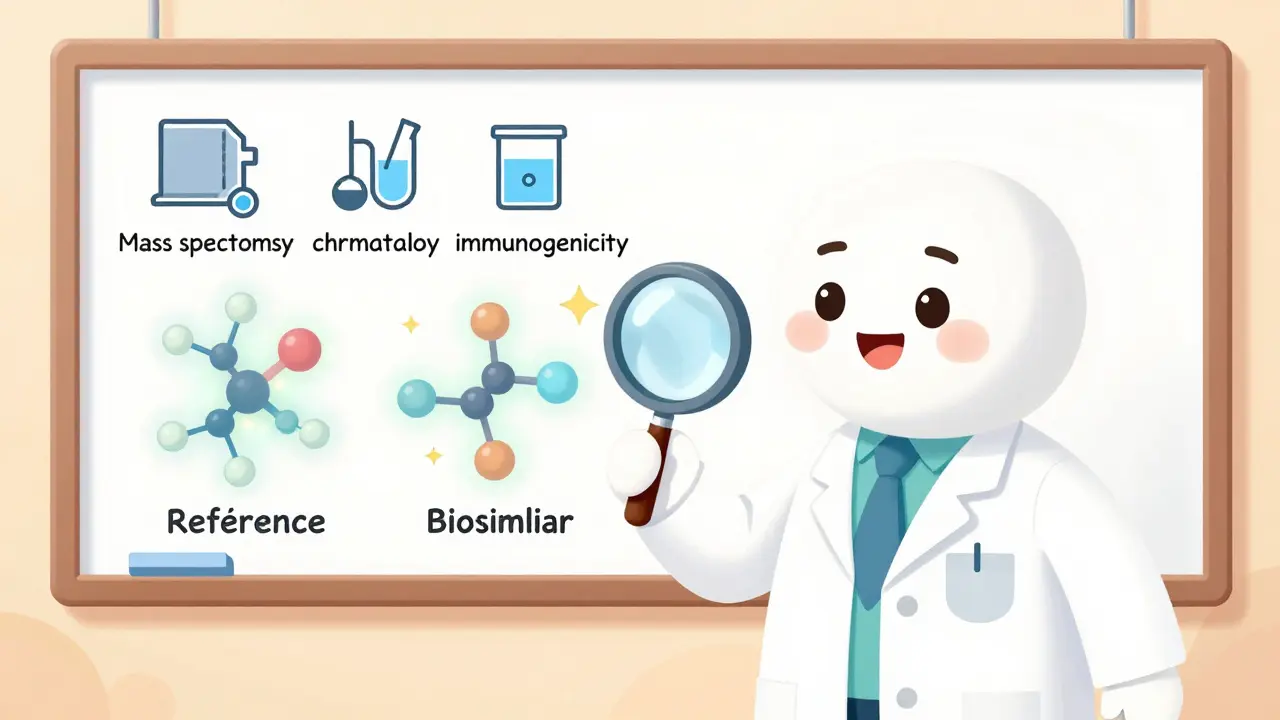
When you hear the word generic, you probably think of a cheap, identical copy of a brand-name pill. But when it comes to biologic drugs - the complex, life-saving treatments for cancer, rheumatoid arthritis, and diabetes - there’s no such thing as an exact copy. That’s where biosimilars are different. They’re not generics. They’re highly similar versions of biologic drugs, made from living cells, not chemicals. And getting them approved by the U.S. Food and Drug Administration (FDA) isn’t just a matter of proving they work the same. It’s a science-heavy, data-driven process that’s changing fast.
Why biosimilars aren’t like generics
Generics are simple. They’re chemically identical to their brand-name counterparts. If you take a generic ibuprofen, you’re getting the exact same molecule as Advil. Biosimilars? Not even close. Biologics are made from living organisms - human or animal cells grown in labs. Their structure is massive, complex, and sensitive to tiny changes in temperature, pH, or manufacturing conditions. Even small differences can affect how the drug works in the body.
That’s why the FDA doesn’t require biosimilar makers to repeat every clinical trial done for the original biologic. Instead, they must prove biosimilarity - meaning the two drugs are so alike in structure, function, and safety that no meaningful difference can be detected. The process is built on three pillars: analytical studies, pharmacokinetic (PK) data, and immunogenicity testing. In plain terms: scientists use advanced tools like mass spectrometry and chromatography to compare the molecular fingerprints of the biosimilar and the reference product. Then they test how fast the body absorbs and clears each drug. Finally, they check for immune reactions - because even a slight change in structure can trigger antibodies that make the drug less effective or cause side effects.
The 2025 FDA overhaul: what changed
Before October 2025, the FDA almost always required a full comparative clinical efficacy study. That meant running trials in patients to prove the biosimilar worked just as well as the original. These studies took years and cost up to $300 million. Many smaller companies couldn’t afford it. As a result, only 76 biosimilars had been approved by the end of 2025 - far fewer than in Europe, where over 100 are on the market.
The FDA’s draft guidance released on October 29, 2025, changed everything. Now, for many biosimilars, you don’t need a clinical efficacy study at all. If three conditions are met - the product is made from a well-characterized cell line, the link between its structure and clinical effect is well understood, and a PK study can be done - then the FDA says analytical and immunogenicity data are enough. This shift is based on advances in technology. Modern tools can now detect differences at the atomic level. If the molecules match down to the last sugar group or amino acid, the FDA believes you can confidently predict how they’ll behave in patients.
For example, drugs like adalimumab (Humira) and trastuzumab (Herceptin) are monoclonal antibodies with well-mapped quality attributes. For these, the new guidance opens the door to faster approvals. But for more complex drugs - like antibody-drug conjugates where a toxic payload is attached to a targeting molecule - the FDA still recommends clinical studies. The science isn’t there yet.
Interchangeability: the big debate
Here’s where things get messy. Interchangeability is the gold standard. It means a pharmacist can swap a biosimilar for the original drug without asking the doctor. In 34 states, laws still require this designation before substitution can happen. But in October 2025, FDA Commissioner Marty Makary made a bold statement: “Every biosimilar should have the designation of interchangeable.”
That’s not how the law works. Under the Biologics Price Competition and Innovation Act (BPCIA), interchangeability is a separate legal category. To earn it, companies had to prove that switching back and forth between the reference product and biosimilar didn’t increase risk. That meant additional “switching studies” - often with dozens of patients cycling between drugs over months. The FDA’s draft guidance eliminated this requirement. Instead, they now say that if a biosimilar meets the standard for biosimilarity, it should be considered interchangeable by default.
But here’s the catch: the FDA hasn’t changed the law. So while they’re now approving biosimilars with interchangeability status without switching studies, pharmacists in many states still can’t substitute without a doctor’s order. The October 2025 approvals of two denosumab biosimilars - Enoby and Xtrenbo - were historic because they were the first time multiple interchangeability designations were granted for the same reference product. But the confusion remains. Doctors are still wary. Pharmacists are stuck in legal limbo. Patients? Many don’t even know the difference.

Who’s making biosimilars - and who’s not
The biosimilar market is dominated by big players. Sandoz leads with 17 approved products. Pfizer and Amgen each have 12 and 10, respectively. But only 12 of the 76 approved biosimilars came from companies with fewer than 100 employees. Why? Because the analytical tools needed - mass spectrometers, high-performance liquid chromatography systems, bioassay labs - cost millions. A small biotech can’t afford to build them from scratch.
That’s why the FDA’s new guidance could be a game-changer. By removing the need for costly clinical trials, the development cost for a biosimilar could drop from $100-300 million to $50-150 million. That’s within reach for more startups. The Biosimilars Council has already seen 87 consultations with small developers since 2023. And the FDA’s Biosimilars Community Resource Center had over 12,000 visitors in October 2025 alone.
But there’s a catch. Even with streamlined pathways, 42% of biosimilar applications still get a “complete response letter” - meaning the FDA asks for more data. That’s because the analytical burden is higher than ever. Companies now have to characterize over 200 quality attributes. If one peak in a chromatogram doesn’t match perfectly, the whole application can stall.
Real-world impact: savings, adoption, and patient experiences
Behind the science are real people. At Mayo Clinic, switching to biosimilars for oncology treatments cut biologic drug costs by 37% - saving $18 million a year. Hospital systems across the U.S. now include biosimilars in 89% of their formularies. But patient awareness? Only 32% know what a biosimilar is.
Patients who’ve switched report mixed results. A September 2025 survey by the Arthritis Foundation of 1,247 users found 78% were satisfied with effectiveness. But 41% had initial safety concerns. Most of those fears faded after talking to their doctor. On Reddit’s r/pharmacy community, 63% of 87 respondents said their biosimilar worked just as well as the original. But 22% noticed more injection site reactions - a common, mild side effect that doesn’t affect long-term outcomes.
Market data shows the U.S. biosimilar market captured only 23% of potential share in 2025 - compared to 67% in Europe. Why? Europe’s regulatory system has been more flexible for years. The European Medicines Agency (EMA) typically requires only one PK study, not full clinical trials. The FDA’s 2025 changes finally start to close that gap. Grand View Research predicts the U.S. market will grow from $18.7 billion in 2024 to $62.3 billion by 2029. McKinsey & Company forecasts biosimilars could capture 40-50% market share by 2030 - potentially saving the healthcare system $150 billion a year.

What’s next? Challenges ahead
Even with the 2025 guidance, roadblocks remain. Patent litigation delays biosimilar entry in 68% of cases, according to the FTC. Drugmakers use legal tactics to block competition - extending patents, filing lawsuits, or paying biosimilar makers to delay launch. These “pay-for-delay” deals still happen, despite FDA efforts.
The interchangeability confusion also lingers. While the FDA says all biosimilars should be interchangeable, the law doesn’t agree. Until Congress updates the BPCIA, pharmacists will keep facing conflicting state laws. And some experts worry. Dr. Paul Baldrick, editor of the Journal of Biological Sciences, warned that long-term, subtle differences might only show up in large populations over years - not in short-term trials.
The FDA’s draft guidance is open for public comment until January 27, 2026. Final rules are expected by June 2026. If they hold, we could see biosimilar approvals jump from 8-10 per year to 15-20. More competition. Lower prices. Better access. But it won’t be automatic. Success depends on clearer laws, more education for doctors and patients, and continued investment in analytical science.
How companies get started now
If you’re a biosimilar developer in 2026, here’s what you need to do:
- Meet with the FDA early - request a Biologics Product Development (BPD) meeting within 30 days of starting development.
- Invest in analytical characterization. Build or partner with a lab that can measure at least 200 quality attributes.
- Design a PK study that matches the reference product’s dosing schedule and route of administration.
- Test immunogenicity in healthy volunteers - look for antibody formation over time.
- Submit your Biologics License Application (BLA) under Section 351(k) of the Public Health Service Act.
- Pay the BsUFA III fees - $1.2 million for the initial application, plus annual fees through September 2027.
The learning curve is steep. It takes 12-18 months just to set up the quality control systems. But with the FDA’s new approach, the finish line is closer than ever.
Are biosimilars the same as generics?
No. Generics are chemically identical copies of small-molecule drugs. Biosimilars are highly similar versions of complex biologic drugs made from living cells. They can’t be exact copies because their structure is too complex. Biosimilars are approved based on extensive analytical and clinical data proving they work the same way - not because they’re identical.
Why do biosimilars cost less than biologics?
Biologics cost $50,000-$100,000 per patient annually because their development takes over a decade and costs $1-$2 billion. Biosimilars don’t need to repeat all the early clinical trials. They rely on data from the original drug and focus on proving similarity. This cuts development time and cost by 50-70%, allowing lower prices.
Can pharmacists substitute biosimilars without a doctor’s approval?
It depends on the state. The FDA now approves biosimilars for interchangeability without requiring switching studies. But 34 states still require a separate interchangeability designation and a doctor’s order before substitution. Only 12 states allow automatic substitution. This patchwork creates confusion for patients and pharmacists.
How long does it take to get a biosimilar approved?
Before 2025, it took 8-10 years and $100-300 million. With the new FDA guidance, it’s now possible to get approval in 5-7 years for $50-150 million. The biggest time-saver is eliminating routine comparative efficacy studies. But analytical characterization still takes 1-2 years to complete properly.
Are biosimilars safe for long-term use?
Yes. Over 100 biosimilars have been used in Europe for nearly two decades with no new safety signals. In the U.S., 76 are approved, and real-world data from hospitals and patient surveys show comparable safety profiles to reference biologics. Minor side effects like injection site reactions are more common but not dangerous. Long-term studies are ongoing, but current evidence strongly supports their safety.